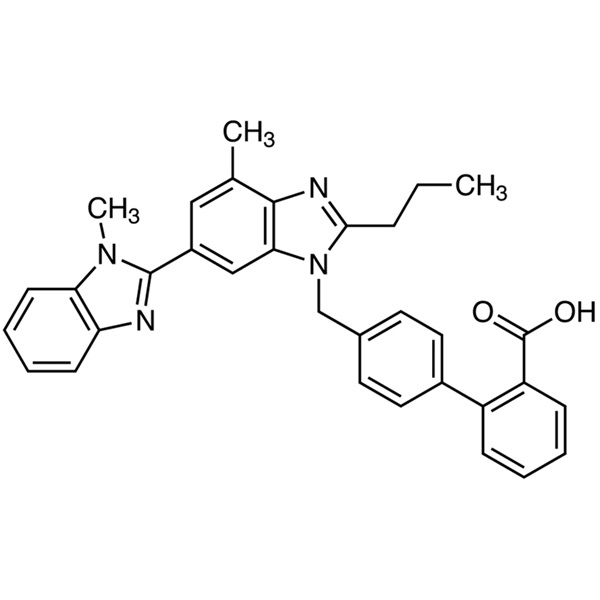
Telmisartan CAS 144701-48-4 Assay 99.0%~101.0% API EP Standard High Quality
Shanghai Ruifu Chemical Co., Ltd. is the leading supplier of Telmisartan (CAS: 144701-48-4) with high quality, can meet the standard of USP / EP. DMF/ CEP/ GMP available.
Ruifu Chemical has been supplying APIs and pharmaceutical intermediates more than 15 years.
Ruifu Chemical can provide worldwide delivery, competitive price, excellent service.
Purchase Telmisartan and related intermediates, please contact us by e-mail: alvin@ruifuchem.com
| Chemical Name | Telmisartan |
| Synonyms | 4'-[[4-Methyl-6-(1-Methyl-1H-Benzimidazol-2-yl)-2-Propyl-1H-Benzimidazol-1-yl]methyl]biphenyl-2-Carboxylic Acid; 4'-[(1,4'-Dimethyl-2'-propyl[2,6'-bi-1H-Benzimidazol]-1'-yl)methyl]-[1,1'-Biphenyl]-2-Carboxylic Acid |
| CAS Number | 144701-48-4 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C33H30N4O2 |
| Molecular Weight | 514.63 |
| Melting Point | 261.0~263.0℃ |
| Water Solubility | Insoluble |
| Stability | Hygroscopic |
| Storage Condition | 2-8℃ |
| COA & MSDS | Available |
| Origin of Product | Shanghai, China |
| Product Categories | API (Active Pharmaceutical Ingredient) |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White or Slightly Yellowish Crystalline Powder | White Crystalline Powder |
| Solubility | Should Meet the Requirement | Conforms |
| IR Identification | Should Correspond to the RS Infrared Absorption | Conforms |
| Appearance of Solution | Should Meet the Requirement | Conforms |
| Related Substances | ||
| Impurity A | ≤0.15% | 0.05% |
| Impurity B | ≤0.15% | 0.02% |
| Impurity C | ≤0.20% | 0.03% |
| Impurity D | ≤0.20% | 0.05% |
| Unspecified Impurities: | ≤0.10% | 0.06% |
| Total Impurities | ≤1.00% | 0.30% |
| Residual Solvents | ||
| Methanol | ≤0.30% | Not Detected |
| Ethanol | ≤0.50% | 0.031% |
| Methylene dichloride | ≤0.06% | Not Detected |
| n-Hexane | ≤0.029% | Not Detected |
| Acetic Acid Ethyl Ester | ≤0.50% | Not Detected |
| Toluene | ≤0.089% | 0.025% |
| Acetic Acid | ≤0.50% | 0.036% |
| Loss on Drying | ≤0.50% | 0.20% |
| Sulphated Ash | ≤0.10% | Conforms |
| Melting Point | 261.0~263.0℃ | 261.5~262.1℃ |
| Particle Size | 90% Less than 200μm | Conforms |
| Assay | 99.0%~101.0% (Dried Substance) | 99.9% |
| Test Standard | Conforms to European Pharmacopoeia 7.0 | Conforms |
Package: Bottle, Aluminium foil bag, 25kg/cardboard drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed. Store in a cool, dry (2-8℃) and well-ventilated warehouse away from incompatible substances. Keep away from sunshine; avoid fire and heat sources; avoid moisture.
Shipping: Deliver to worldwide by air, by sea, by FedEx / DHL Express. Provide fast and reliable delivery.

Hazard Symbols Xi - Irritant
Risk Codes 36/37/38 - Irritating to eyes, respiratory system and skin.
Safety Description S22 - Do not breathe dust.
S24/25 - Avoid contact with skin and eyes.
S36 - Wear suitable protective clothing.
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
WGK Germany 2
RTECS DV2037500
HS Code 2933990099
Telmisartan (CAS: 144701-48-4) is a benzimidazole derivative and a non-peptide angiotensin II receptor antagonist with antihypertensive property. Telmisartan selectively antagonizes angiotensin II binding to the AT1 subtype receptor, located in vascular smooth muscle and adrenal gland. The antagonism results in vasodilation and inhibits the angiotensin II-mediated aldosterone production, which in turn leading to a decrease in sodium and water as well as an increase in potassium excretion leading to a subsequent reduction in blood pressure. Telmisartan is a new type of blood pressure drugs, is used alone or in combination with other classes of antihypertensives for the treatment of hypertension. Telmisartan was originally formulated by the German pharmaceutical company Boehringer Ingelheim; it earned the German patent EP502,314 in 1991, was first approved to enter the American market in November 1998, and then entered German, Philippines, Australian, Belgium, British, and other markets.
C33H30N4O2
Mr 514.6
[144701-48-4]
DEFINITION
4′-[[4-Methyl-6-(1-methyl-1H-benzimidazol-2-yl)-2-propyl-1H-benzimidazol-1-yl]methyl][1,1′-biphenyl]-2-carboxylic acid.
Content: 99.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance: white or slightly yellowish, crystalline powder.
Solubility: practically insoluble in water, slightly soluble in methanol, sparingly soluble in methylene chloride. It dissolves in a 40 g/L solution of sodium hydroxide R.
It shows polymorphism (5.9).
IDENTIFICATION
Infrared absorption spectrophotometry (2.2.24).
Comparison: Telmisartan CRS.
If the spectra obtained in the solid state show differences, dissolve the substance to be examined and the reference substance separately in hot anhydrous ethanol R, evaporate to dryness and record new spectra using the residues.
TESTS
Appearance of solution. The solution is not more intensely coloured than reference solution Y4 (2.2.2, Method II).
Dissolve 0.5 g in a 40 g/L solution of sodium hydroxide R and dilute to 10 mL with the same solvent.
Related substances. Liquid chromatography (2.2.29).
Test solution. To 25 mg of the substance to be examined add about 5 mL of methanol R and 100 μL of a 40 g/L solution of sodium hydroxide R. Dissolve using sonication and dilute to 50.0 mL with methanol R.
Reference solution (a). Dilute 1.0 mL of the test solution to 10.0 mL with methanol R. Dilute 1.0 mL of this solution to 100.0 mL with methanol R.
Reference solution (b). Dissolve the contents of a vial of telmisartan for system suitability CRS (containing impurities A, B, C, E and F) in 2 mL of methanol R.
Reference solution (c). To 5 mg of telmisartan for peak identification CRS (containing impurity D) add about 5 mL of methanol R and 100 μL of a 40 g/L solution of sodium hydroxide R. Dissolve using sonication and dilute to 10 mL with methanol R.
Column:
– size: l = 0.125 m, Ø = 4.0 mm;
– stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 μm) with a pore size of 10 nm;
– temperature: 40 °C.
Mobile phase:
– mobile phase A: dissolve 2.0 g of potassium dihydrogen phosphate R and 3.8 g of sodium pentanesulfonate monohydrate R1 in 900 mL of water for chromatography R, adjust to pH 3.0 with dilute phosphoric acid R and dilute to
1000 mL with water for chromatography R;
– mobile phase B: methanol R1, acetonitrile for chromatography R (20:80 V/V);
Time (min) Mobile phase A (per cent V/V) Mobile phase B (per cent V/V)
0 - 3 70 30
3 - 28 70 → 20 30 → 80
Flow rate: 1 mL/min.
Detection: spectrophotometer at 230 nm.
Injection: 10 μL.
Identification of impurities: use the chromatogram supplied with telmisartan for system suitability CRS and the chromatogram obtained with reference solution (b) to identify the peaks due to impurities A, B, C, E and F;
use the chromatogram supplied with telmisartan for peak identification CRS and the chromatogram obtained with reference solution (c) to identify the peak due to impurity D.
Relative retention with reference to telmisartan
(retention time = about 15 min): impurity A = about 0.2;
impurity E = about 0.6; impurity F = about 0.7;
impurity B = about 0.9; impurity C = about 1.5;
impurity D = about 1.6.
System suitability: reference solution (b):
– the chromatogram obtained with reference solution (b) is similar to the chromatogram supplied with telmisartan for system suitability CRS;
– resolution: minimum 3.0 between the peaks due to impurity B and telmisartan.
Limits:
– impurities C, D: for each impurity, not more than twice the area of the principal peak in the chromatogram obtained with reference solution (a) (0.2 per cent);
– impurities A, B: for each impurity, not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.15 per cent);
– unspecified impurities: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (0.10 per cent);
– total: not more than 10 times the area of the principal peak in the chromatogram obtained with reference solution (a) (1.0 per cent);
– disregard limit: 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.05 per cent).
Loss on drying (2.2.32): maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105℃.
Sulfated ash (2.4.14): maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.190 g in 5 mL of anhydrous formic acid R. Add 75 mL of acetic anhydride R. Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M perchloric acid is equivalent to 25.73 mg of C33H30N4O2.
IMPURITIES
Specified impurities: A, B, C, D.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use): E, F, G, H, I, J.
A. 4-methyl-6-(1-methyl-1H-benzimidazol-2-yl)-2-propyl-1H-benzimidazole,
B. 4′-[[7-methyl-5-(1-methyl-1H-benzimidazol-2-yl)-2-propyl-1H-benzimidazol-1-yl]methyl][1,1′-biphenyl]-2-carboxylic acid,
C. tert-butyl 4′-[[4-methyl-6-(1-methyl-1H-benzimidazol-2-yl)-2-propyl-1H-benzimidazol-1-yl]methyl][1,1′-biphenyl]-2-carboxylate,
D. unknown structure,
E. 1-[(2′-carboxy[1,1′-biphenyl]-4-yl)methyl]-4-methyl-2-propyl-1H-benzimidazole-6-carboxylic acid,
F. 4′-[[4-methyl-6-(1-methyl-1H-benzimidazol-2-yl)-2-propyl-1H-benzimidazol-1-yl]methyl][1,1′-biphenyl]-2-carboxamide,
G. 4′-[[4-methyl-6-(1-methyl-1H-benzimidazol-2-yl)-2-propyl-1H-benzimidazol-1-yl]methyl][1,1′-biphenyl]-2-carbonitrile,
H. tert-butyl 4′-(bromomethyl)[1,1′-biphenyl]-2-carboxylate,
I. methyl 4′-[(1,7′-dimethyl-2′-propyl-1H,3′H-[2,5′-bibenzimidazol]-3′-yl)methyl][1,1′-biphenyl]-2-carboxylate,
J. 4′-[(5-chloro-1,7′-dimethyl-2′-propyl-1H,3′H-[2,5′-bibenzimidazol]-3′-yl)methyl][1,1′-biphenyl]-2-carboxylic acid.
-
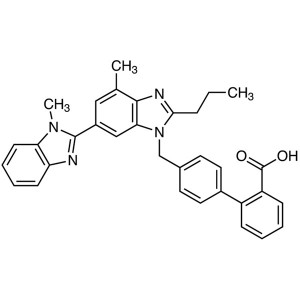
Telmisartan CAS 144701-48-4 Assay 99.0%~101.0% ...
-
2-(p-Tolyl)benzoic Acid CAS 7148-03-0 Purity >9...
-
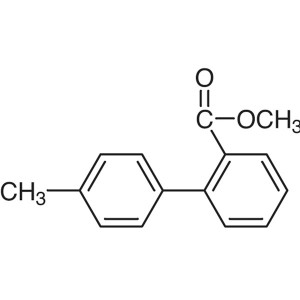
Methyl 2-(p-Tolyl)benzoate CAS 114772-34-8 Puri...
-
![Methyl 2-[4-(Bromomethyl)phenyl]benzoate CAS 114772-38-2 Telmisartan Intermediate](https://www.ruifuchem.com/uploads/Methyl-2-4-Bromomethylphenylbenzoate-CAS-114772-38-2-Purity-99.0-HPLC-Telmisartan-Intermediate-Factory-300x300.jpg)
Methyl 2-[4-(Bromomethyl)phenyl]benzoate CAS 11...
-
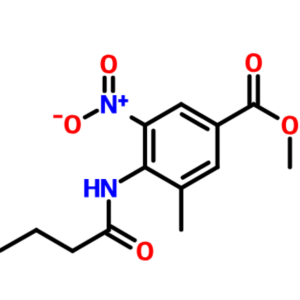
Methyl 4-(Butyrylamino)-3-Methyl-5-Nitrobenzoat...
-
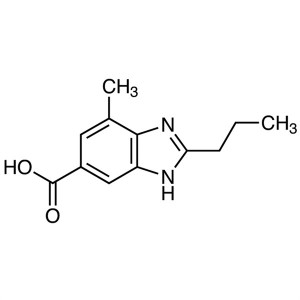
Telmisartan Benzimidazole Acid CAS 152628-03-0 ...
-
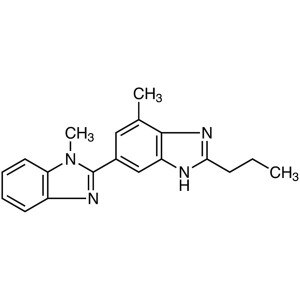
Telmisartan Intermediate CAS 152628-02-9 Purity...
-
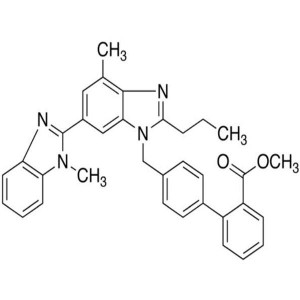
Telmisartan Methyl Ester CAS 528560-93-2 Purity...
-
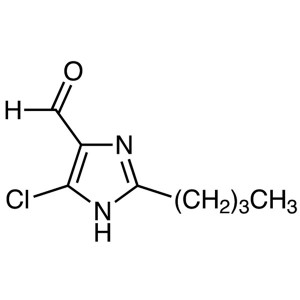
2-Butyl-4-Chloro-5-Formylimidazole (BCFI) CAS 8...
-
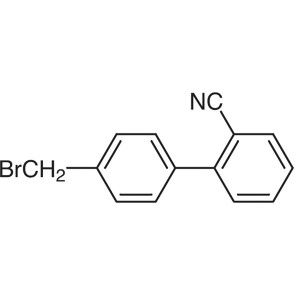
4′-Bromomethyl-2-Cyanobiphenyl (Br-OTBN) ...
-
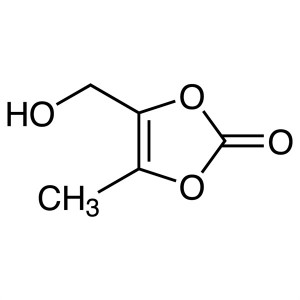
DMDO-OH CAS 91526-18-0 Purity >97.0% (HPLC) Azi...
-
Losartan Potassium CAS 124750-99-8 API Factory ...
-
Valsartan CAS 137862-53-4 Assay 98.0~102.0% API...
-
Olmesartan Medoxomil CAS 144689-63-4 Purity >99...
-
DMDO-Cl CAS 80841-78-7 Purity >96.0% (GC) Olmes...
-
Diethyl 2-Propyl-1H-Imidazole-4,5-Dicarboxylate...